Readers with an interest in process safety (isn't that everyone?) should be aware of some important limitations in the traditional method for obtaining TMR, the time to maximum rate, as referenced here, for example.
Wilfried Hoffmann, one of our principal consultants supporting users and an experienced former specialist in process safety at Pfizer, highlighted in his December 2014 DynoChem webinar how:
You can watch a preview of Wilfried's discussion on YouTube. You can also read more in his book chapter here.
Needless to say, we recommend that you use kinetic information to calculate TMR, so that you can make stronger safety statements. If you have access to DynoChem and our online library, follow this link to find the main tools, step by step training and a nice customer case study by Siegfried.
Wilfried Hoffmann, one of our principal consultants supporting users and an experienced former specialist in process safety at Pfizer, highlighted in his December 2014 DynoChem webinar how:
- the traditional approach to TMR using MTSR (maximum temperature reached as a result of adiabatic temperature rise of the desired reaction, after a cooling failure) ignores the kinetics of the desired reaction; that makes it simple, but potentially less accurate; this is understandable as when the method was developed, kinetics were less readily obtained
- the traditional method neglects the time to MTSR in calculating TMR and time to explosion
- the modern method uses kinetics to get the true TMR
- there are two extremes where the difference between traditional and modern methods is significant:
- with a slow reaction, perhaps taking place at low temperature, it may take a long time to reach MTSR; in this case, traditional TMR < true TMR and the traditional method may be used safely; it overestimates risk
- in situations where on the way to MTSR, there is a heat flow contribution from the undesired reaction, the adiabatic temperature rise will then be higher than MTSR and the true TMR will be shorter than the estimate using the traditional method.
You can watch a preview of Wilfried's discussion on YouTube. You can also read more in his book chapter here.
Needless to say, we recommend that you use kinetic information to calculate TMR, so that you can make stronger safety statements. If you have access to DynoChem and our online library, follow this link to find the main tools, step by step training and a nice customer case study by Siegfried.
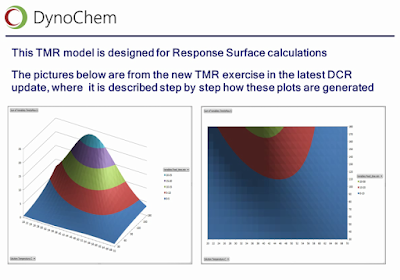 |
| Slide from Wilfried Hoffmann's webinar, illustrating response surfaces of true TMR, obtained from kinetic models of the desired and undesired reactions. |
