Many thanks to customers who engaged with Scale-up Systems as we "built, broke and bettered" our biologics model library in the run-up to release late last year.
More than one hundred biopharmaceutical companies in the Scale-up Suite global user community can now access the tools for immediate use here (https://dcresources.scale-up.com/?q=bio). An overview of the biologics library is available here.
We expect each tool to grow and be refined by the repeated use that is typical of customer activity and we look forward to supporting more users in taking up the tools in their daily work.
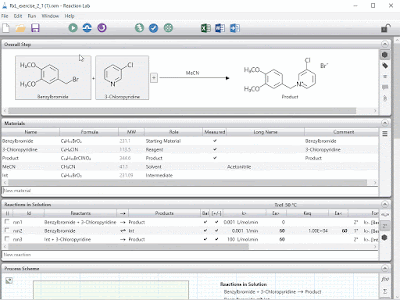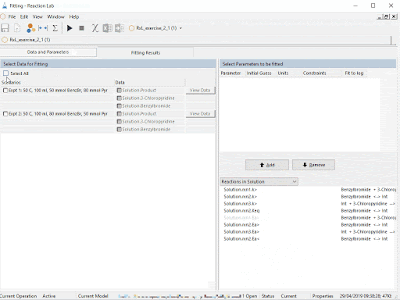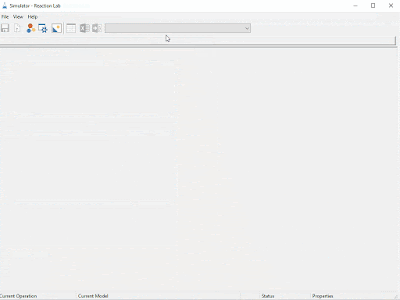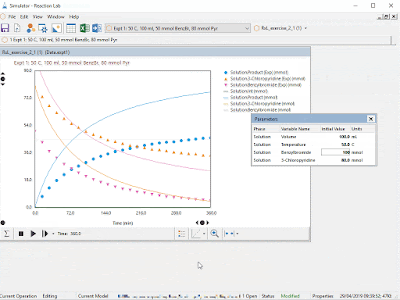
Much like the small molecule opportunity, mechanistic modeling has great potential to accelerate the development of large molecules by shortening development time, making best use of experiments and anticipating manufacturing challenges. Ours is the first fit-for-purpose and comprehensive mechanistic model library to be built and released in this space, another first of which we are very proud.
 |
| Using the Dynochem biologics library delivers daily benefits in development and scale-up while creating digital twins to support your digitalization strategy |
Training opportunities using the new tools will be available at regular intervals this year. Let us know if you'd like a dedicated session for your company or site.
Feel free to share this post with anyone you think may benefit.